In the process of agricultural development, nitrogen fertilizer is crucial to improving crop yields. Among the numerous nitrogen fertilizers, ammonium chloride (NH₄Cl) stands out for its unique chemical properties and affordable advantages. However, using ammonium chloride is like a double-edged sword: using it well can increase production, but using it wrong will seriously damage the soil. This article will introduce in detail the basic knowledge of the use of ammonium chloride in agriculture, scientific usage methods, and how to protect the ecological environment while using it.

The importance of ammonium chloride in agriculture
Affordable nitrogen source

Ammonium chloride usually contains between 24% and 26%, which is cheaper than urea (46% nitrogen content), and can continuously and stably release nitrogen. This makes it a favorite among many farmers, especially suitable for growing fast-growing vegetables with short growth cycles. Data shows that with the same amount of nitrogen, ammonium chloride costs 15% – 20% less than ammonium sulfate. What is more convenient is that crops can directly absorb ammonium chloride without requiring complex transformation processes.
Double nutritional advantages
In addition to providing nitrogen, ammonium chloride can also bring special benefits to some crops:
•Enhance stress resistance: Chlorine can improve the drought resistance of crops such as wheat and corn. For example, in water-deficient areas, wheat fertilized with ammonium chloride grows better and has a higher survival rate.

•Inhibit diseases: Ammonium chloride can effectively reduce diseases such as potato scabs, coconut heart rot. In potato planting, the chance of scab disease is significantly reduced after using ammonium chloride as fertilizer.
Good physical characteristics
The crystal particles of ammonium chloride are not easy to accumulate, it is more convenient to spread fertilizer, and the machine operation is also easy. Moreover, it is easy to dissolve in water, which is especially suitable for drip irrigation systems, and can help farmers who grow vegetables on a large scale save a lot of manual fertilization costs.
Applicable crops and usage precautions
Suitable for use
•Chlorine-resistant crops: Rice, cotton, spinach and other crops are not afraid of chlorine. In rice fields, chlorine can help rice perform photosynthesis, making rice grow strong and yield high.
| Stage | Specific process | The role of chlorine element |
| Photoreaction | Light energy is absorbed by photosynthetic pigments, which decompose water into oxygen and [H], while ADP binds with Pi to generate ATP | Participate in maintaining the structure and function of chloroplasts, ensuring the normal functioning of photosynthetic pigments, and improving the efficiency of light reactions; Adjusting the opening and closing of stomata, controlling the absorption of carbon dioxide, indirectly affecting the light reaction |
| Dark reaction | Carbon dioxide combines with a five-carbon compound to form two molecules of a three- carbon compound; Under the action of ATP and [H], a three- carbon compound is reduced to form organic compounds (CH₂O), while the original compound is regenerated | Helps maintain cellular ion balance, creates a suitable environment for dark – reaction enzymes, and promotes smooth dark reactions. |
| Final Effect | Through light and dark reactions, light energy is converted into chemical energy and stored in organic matter, providing energy and material basis for the growth of rice | Improve the efficiency of photosynthesis in rice, enabling it to synthesize more organic matter, resulting in robust growth and increased yield |
•Short-term crops: After lettuce absorbs chloride ions, the cells divide faster, grow faster and better, and keep them fresh for longer after picking.
•Improvement of saline-alkali land: In coastal saline-alkali land, using ammonium chloride and calcium fertilizer can replace the sodium ions in the soil, improve soil conditions, and allow crops to grow better.
Caution or prohibition of use
•Avoid chlorine crops: Ammonium chloride cannot be used for tobacco and citrus. If ammonium chloride is used, the tobacco will have a worse burning property and the citrus leaves will become scorched, affecting the quality of agricultural products.
•Arid areas: There is less rainfall in arid areas. After using ammonium chloride, the chloride ions cannot be leached, which will accumulate more and more in the soil. Over time, it will damage the root system of the crop.
•Facility agriculture: Be careful when using ammonium chloride in closed environments such as greenhouses, as it may release chlorine, causing poor air quality in the shed and damaging crops.
Scientific usage methods
The amount of ammonium chloride should be determined based on the chlorine content in the soil. You can refer to this formula:
Ammonium chloride dosage (kg/mu) = (Nitrogen required for target yield × 0.4) ÷ 0.25
(Note: 0.4 is the nitrogen utilization rate of ammonium chloride. The specific value can be adjusted according to the soil detection results)
For best results, you can also use it in combination like this:
•In rice fields, using ammonium chloride and silicon fertilizer can make the rice stems stronger;
•In orchards, mixing ammonium chloride and humic acid can reduce the effects of chloride ions;
•When growing vegetables in a greenhouse, alternately use ammonium chloride and ammonium nitrate to enhance vegetable growth.
Additional considerations include: 10-15 days before sowing, apply ammonium chloride as base fertilizer to the soil; top-dressing for leafy vegetables should be prevented from being washed away by rain after the dew dries in the morning; do not use ammonium chloride during the fruit expansion period and 30 days before harvest, as this can ensure the quality of the fruit.
Ecological risks and sustainable solutions
Three long-term risks
•Soil acidification: If you use ammonium chloride for a long time, the pH of the soil will drop by 0.2 – 0.5 every year. Over time, the soil becomes too acidic and some crops will not grow well.
•Secondary salinization: When the chloride content in the soil exceeds 200 mg/kg, secondary salinization will occur, soil fertility will decrease, and crop yield will also decrease.
•Microbial imbalance: Ammonium chloride will reduce the activity of nitrifying bacteria in the soil by more than 40%, destroying the soil microbial balance, affecting nutrient circulation and crop health.
Solutions
•Biochar amendment: Using ammonium chloride and biochar (pH8-10) made of straw can neutralize soil acidity. Some experimental fields have improved soil acidification problems through this method.
•Adsorbent materials: Use zeolite or bentonite and other materials to absorb excess chloride ions in the soil and improve soil quality.
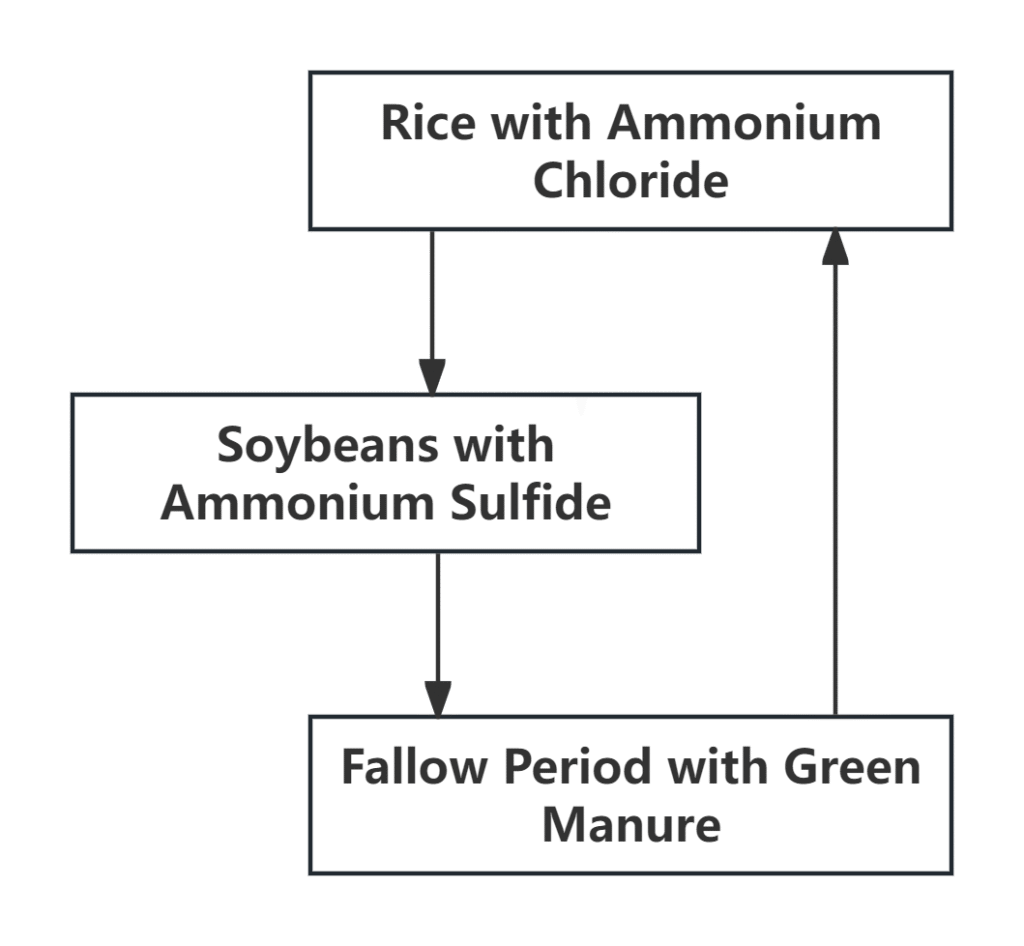
•Crop rotation: Use reasonable crop rotation methods, such as ammonium chloride for rice and ammonium sulfide for soybeans, and then plant green manure and fallow in the middle, which can restore the soil to health.
Crop rotation system flow chart:
Frontier Exploration
Breakthrough in sustained release technology
Coating ammonium chloride with a polylactic acid coating can make the rate of chloride ions release consistent with the crop’s demand for chlorine, reducing fertilizer waste and loss.
Functional compound fertilizer
“Maxidonin chloride” is a fertilizer that mixes ammonium chloride and seaweed extract. In Hainan banana cultivation, the yield increased by 23% after using this fertilizer, and the banana’s stress resistance has also become stronger.
Digital management
Israel’s Agritask platform monitors chloride ion changes in real time through soil sensors, automatically generates a fertilization reminder map, helping farmers to apply fertilizers accurately, which is both cost-effective and environmentally friendly.
Conclusion
Ammonium chloride’s role in agriculture reflects the delicate balance between human practices and nature. In modern, sustainable agriculture, it’s essential to leverage its nitrogen – rich benefits while minimizing chloride – related risks. As the farming adage goes, “No fertilizer is inherently good or bad; it’s all about proper use”—a principle perfectly demonstrated by ammonium chloride.
